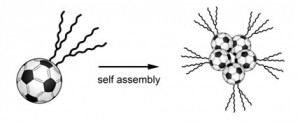
 Researchers are attaching chains of carbon atoms to C60 ‘buckyball’ molecules causing them to controllably self-assemble into spheres, wires and sheets. The strings are semiconductive and photoconductive, and the structures could improve doping in organic solar cells.
Researchers are attaching chains of carbon atoms to C60 ‘buckyball’ molecules causing them to controllably self-assemble into spheres, wires and sheets. The strings are semiconductive and photoconductive, and the structures could improve doping in organic solar cells.
Once the chains are attached, the resulting molecule is an ‘amphiphile’ (see box below) – its ends have a different affinity to certain solvents, much like washing-up detergent in water.
Detergents are well understood, and are increasingly being exploited to create order in liquid, forming ‘soft’ structures, some of which can used as the basis for solid structures.
“We have applied science that is normally applied to detergent, and applied it to molecules you wouldn’t normally expect it to apply to,” Dr Martin Hollamby of Keele University told Electronics Weekly.
This means that self-assembly techniques developed using detergents can be adapted for C60.
Hollamby is working with Dr Takashi Nakanishi of the Japanese National Institute for Materials Science.
In their experiments, the chains attached to C60 are branched alkanes (a form of hydrocarbon).
When dissolved in a liquid n-alkane (octane is an alkane eight carbon atoms long, so n=8), the new molecules assemble to form a spherical core of C60 molecules within a shell of carbon chain tails (see box below).
“Changing the chemistry of the chains can lead to gels made of bundled C60 wires that have a measureable photoconductivity,” said Hollamby. “By adding pristine C60 in place of the solvent, we instead prepare a sheet-like material now with totally different properties.”
The wires are hexagonally-packed gel-fibres containing insulated C60 nanowires, according to a Nature Chemistry paper on the work (‘Directed assembly of optoelectronically active alkyl–π-conjugated molecules by adding n-alkanes or π-conjugated species‘).
“The assembled structures contain a large fraction of opto-electronically active material and exhibit comparably high photo-conductivities. This method is shown to be applicable to several molecules, and can be used to construct organised functional materials,” said the paper.

Neutron beamline D11 at the Institut Laue-Langevin (ILL) allows scientists to watch molecular self-assembly
Many different structures can be produced by making small changes to the chemical structure and the additives (solvent or C60) used, according to the team, and this level of control over self-assembly in complex molecules such as C60 is not accessible by any other method reported to date.
So what has all this got to do with electronics?
Importantly for electronics, C60 is a strong electron acceptor, and is already used to dope organic solar cells.
However, according to Hollamby, unstructured C60 is an insulator and only becomes a semiconductor if C60s are bought in close proximity, as they are in the amphiphilic wires, for example. And the wires come ready-insulated by being surrounded by the alkane tails.
What Keele and the National Institute for Materials Science have done is put another tool in the toolbox of organic electronics research. Dots, wires and sheets of C60 are now available for experimentation – although currently only in solution.
Already Hollamby is trying to make solar cells and capacitors using the structures.
The neutron scattering facility (Beamline D11, see photo) at the Institut Laue-Langevin (ILL) was used to investigate assembly and resulting structures.
“The light elements that makes up these molecules are easily located by neutrons” said Dr Isabelle Grillo at ILL.”Small-angle neutron scattering which we use at the ILL allows us to characterise the self-assembled systems from the nanometre scale to tenth of micrometres and observes the coming together of C60s into beautiful core structures.”
|
Amphiphile? In chemistry, ‘amphiphile’ is used to describe a molecule, like washing-up detergent, where one end is attracted to water (hydrophilic) and the other end is repelled by water (hydrophobic) or is attracted to fat (lipophilic). The name only really makes sense in the latter case – the molecule is attracted to more than one thing, so it is ambiphilic – but is used for both.As there is no ‘hydro’ in the term, chemists can borrow it to describe a molecule which has one end attracted to an arbitrary solvent, and the other end is repelled by the solvent, or attracted to something else. In the case of a C60 molecule with an alkane tail, the alkane is attracted to alkane solvent and the C60 is repelled by the solvent. As it happens, both ends of the molecules are repelled by water, leading the researchers to dub them ‘hydrophobic amphiphiles’. Several stable structures form spontaneously when amphiphiles are dissolved in their chosen solvent. ‘Micelles’ are spherical, with the phobic ends of many molecules gathered together with a sphere of tails sticking out. Wires, sometimes called nanowires, have a line of phobic ends surrounded by a tube of solvent-philic ends. Sheets, called a lamellar mesophases, are like a sandwich – with a double-layer 2d mat of phobic ends sandwiched between two mats of philic ends. More complex forms include a hollow sphere consisting of a double-layer spherical shell of phobic ends with philic ends coating both the outside and the inside. |